Generation of Uterine Mini-organ (Endometrial Organoids)
I was fortunate to be one of the main researchers at Northwestern University, Chicago, IL, that developed a novel technique to generate uterine mini-organs (endometrial organoids). Using this technique that I developed there, we will generate these organoids using uterine biopsies from patients already undergoing routine hysterectomy (hence there is no added risk for our volunteers). We will isolate cells for the inner layer of the uterus (the endometrium) and culture them in specific conditions that allow these cells to form organoids. To mimic the true physiology of the human body, we will supplies these organoids with varying levels of hormones (estrogen, progesterone and testosterone) similar to the hormonal changes during a full 28-day menstrual cycle. With this method, we have validated that these endometrial organoids in fact can respond to these hormones just like the real organ in the human body.
A recent study has reported that Zika virus can infect uterine endometrial cells. We will use our endometrial organoid system to dissect the mechanism of Zika virus infection in the uterus and how it can pass on to the infant. Furthermore, we will use the organoids to test previously reported factors with Zika neutralization properties to see if they can stop the spread of Zika virus infection within the endometrial organoids and block its further transmission.
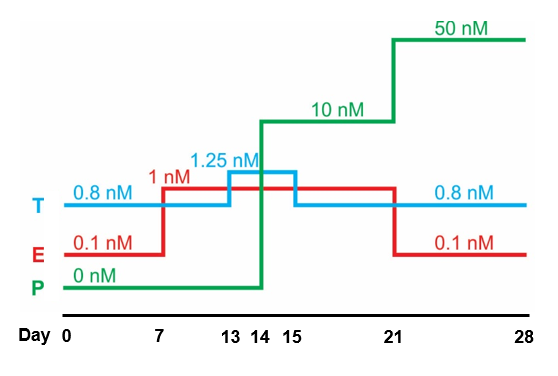
A diagram illustrating the different levels of estrogen (E), progesterone (P) and testosterone (T) during a full 28-day menstrual cycle. We will grow our uterine organoids in these conditions to mimic the true physiology of the human body in labolatory. This culture condition is based on our team's previously studies at Northwestern University: Arslan et al., 2015. Endocrinology. and Wiwatpanit et al., 2019. The Journal of Clinical Endocrinology & Metabolism.
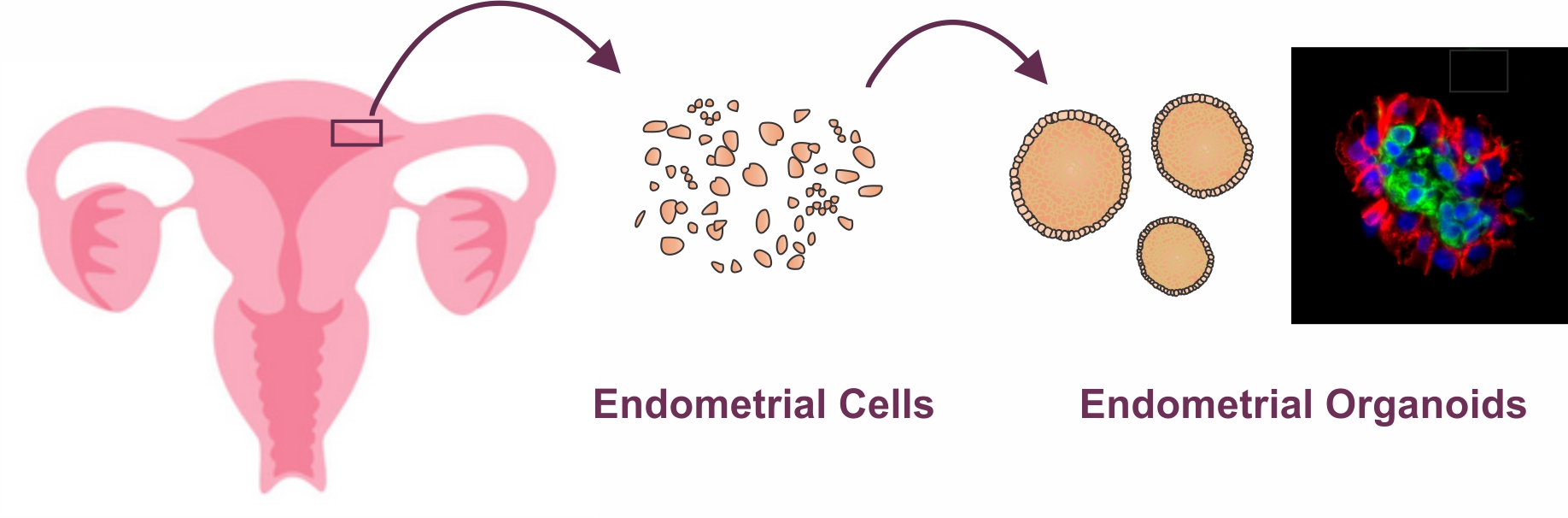 An illustration showing overall steps of generating uterine endometrial organoids from uterine bipsies from volunteers who are already undergoing routine hysterectomy. For detailed protocol and video tutorial on how to generate scaffold-free endometrial organoids, please visit Murphy AR, Wiwatpanit T et al., 2019 *
An illustration showing overall steps of generating uterine endometrial organoids from uterine bipsies from volunteers who are already undergoing routine hysterectomy. For detailed protocol and video tutorial on how to generate scaffold-free endometrial organoids, please visit Murphy AR, Wiwatpanit T et al., 2019 *
*no graphic images, but there are pictures of tissue biopsies
- Published on Jan 15, 2020
- 18 views
- 0 comments
- Print this page
- Back to Methods